Shellfish Hatcheries Adapt as Ocean Becomes More Acidic
by Laurie Schreiber

Bill Mook, Mook SeaFarms with juvenile (seed) oysters in the hatchery.
Ocean acidification (OA) has been impacting shellfish aquaculture operations throughout the United States, by making the shells weaker and lowering larval production.
As a result, hatcheries have been monitoring the situation and implementing techniques to mitigate impacts.
During a Northeast Coastal Acidification Network Industry Webinar, held Sept. 18, Alan Barton, production manager for Whiskey Creek Shellfish Hatchery in Tillamook, Ore., said that, over the past decade, the Pacific Northwest shellfish industry has been grappling with the effects of ocean acidification on commercial production of Pacific oyster larvae.
According to a news release provided before the webinar, the region is characterized by strong summertime upwelling, which brings naturally acidic deeper ocean water up onto the Oregon and Washington continental shelf. As such, the region is already “on the edge” of the saturation state threshold required for normal larval shell development. The added impacts of human-induced acidification observed over the past decade pushed coastal bays over the threshold, leading to devastating seed production failures from 2007 to 2009.
“Upwelling” is a process in which deep, cold water rises toward the surface, according to the National Ocean Service.
Carbon chemistry affects
larval shell formation
in natural environments.
“Our industry has made a great deal of progress in understanding, and adapting to OA in recent years, and has restored much of our historic production through coordinated monitoring up and down our coastline along with development of treatment systems in commercial hatcheries,” Barton said in the release. “These efforts have not only allowed us to address the direct effects of OA on initial larval shell development, but have greatly improved our understanding of secondary and tertiary factors affecting production, as the advance of OA in our region alters the way our coastal ecosystems function.”
During the webinar, Barton explained that Whiskey Creek is a major supplier of larvae to shellfish farms in the Pacific Northwest. The industry there supports 3,000 jobs and contributes $300 million to the economy, he said. Carbon chemistry affects larval shell formation in natural environments, he said. “We started having trouble a decade ago,” Barton said. “It’s a problem for people who depend on our product.”
The problem became apparent in 2007, when prolonged larval mortality at Whisky Creek led to near zero production that year, he said. Uncertain of the cause, the hatchery studied possible bacterial and water quality issues, but was unable to find the source of the problem, he said. The hatchery experienced a huge mortality event in July 2008, that wiped out larvae, he said. The event coincided with a major upwelling along the coast.
“We started to think about carbon chemistry as a cause of the problem,” Barton said.

Seed (spat) oysters with a quarter for size comparison.
In the summer of 2009, the hatchery performed a comprehensive monitoring program in collaboration with Oregon State University’s Burke Hales Laboratory, he said. With the help of that study, he said, they were able to define benchmarks for data quality, figure out when to time spawning, and develop an automated buffering system. As a result, most of the direct effects of carbon dioxide on larvae were eliminated. The buffering process adds alkaline chemicals to balance acidic chemistry.
“Ultimately, this has helped us think of our hatchery as part of a system,” Barton said. “There’s a lot more CO2 in the atmosphere than there used to be, and it’s causing problems for the hatchery – not just for us but around the world….By thinking about where the problems come from, we can better tailor solutions in the hatchery.”
Ultimately, he added, the cause of the problem is fossil fuel emissions.
Bill Mook, owner of Mook Sea Farm, an oyster farm and hatchery in Walpole, said his operation began experiencing larval production problems in 2009. In the news release, he said, the problems included the occasional failure of fertilized eggs to become viable larvae, but more often, prolonged larval durations.
“Larval production was highly unpredictable,” he said in the release. “West Coast hatcheries had recently experienced similar larval production problems, which were determined to be caused by decreased calcium carbonate saturation states in their incoming water. In contrast to the West Coast, our water’s decreased saturation states resulted from a combination of increased atmospheric CO2 and increased freshwater runoff from heavy precipitation events.”
This has helped us
think of our hatchery
as part of a system.
– Alan Barton.
Mook subsequently installed systems to buffer seawater used for larval and juvenile production in the hatchery, and restored production to better than pre-2009 levels.
“We use inexpensive pH meters and controllers to maintain the pH in our hatchery culture systems,” he said in the release. “Along with increasing atmospheric CO2, precipitation in the Northeast is projected to increase in spring and fall months. While we can control seawater chemistry in the hatchery, many questions remain about the extent to which coastal acidification will affect juvenile and market oyster grow out at our lease site. In 2014, working with Dr. Joe Salisbury of UNH we installed sophisticated seawater monitoring equipment to help us answer some of these questions.”
During the webinar, Mook explained that 2009 was a very wet year, and he initially attributed larval production problems to that year’s large rainstorms with a lot of runoff.
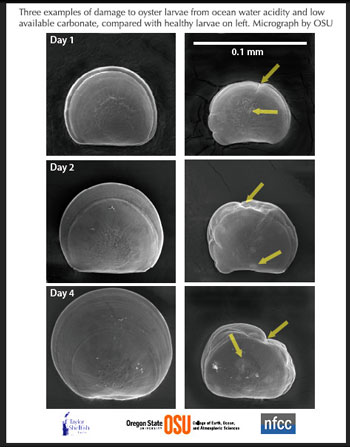
Pacific oyster larvae from the same spawn, raised by the Taylors Shellfish Hatchery in natural waters of Dabob Bay, WA having favorable (left column, pCO2 = 403 ppm, Waragonite = 1.64, and pH (total) = 8.00) and unfavorable (right column, pCO2 = 1418 ppm, Waragonite = 0.47, and pH (total) = 7.49) carbonate chemistry during the spawning period. Similarly unfavorable water conditions occur at Dabob Bay and Netarts Bay, OR, due to regional upwelling of high pCO2 waters to the surface. Images are Scanning Electron Microscopy (SEM) of representative larval shells from each condition from 1 to 4 days post-fertilization. As the sampling is destructive, each larva shown is a different organism, and should not be interpreted as the same larvae ageing through time. Under more acidified conditions, right column, development of shell is impaired; arrows show defects (creases) and some features (light patches on shell) that are suggestive of dissolution. The scale bar in the upper right panel is 0.1 mm, or approximately the diameter of a human hair. Photo credit: Brunner/Waldbusser.
“On occasion we saw no success after egg fertilization,” he said. Plus, on occasion, they saw cessation of larval feeding, poor egg conversion, and poor growth, with larvae taking three to four weeks to grow to the juvenile stage, versus a typical 14 to 16 days.
Buffering the sea water has resulted in fast larvae growth, high survival rates, and high conversion to juveniles, he said.
The backdrop to the problem, Mook said, is the rapid increase of carbon dioxide, since the industrial revolution, which is dissolving in the oceans. “But we observed a lot of these problems occurring during big rain events,” which have been increasing in frequency along the Maine coast, he said. In addition, an increase in dissolved organic matter in the ocean, due to runoff, is blocking sunlight and therefore reducing productivity, he said. “There may be other things as well,” he said “But it has a great deal of bearing on what we think is going on, in terms of carbonate chemistry.”
Buffering at his hatchery has solved larval production problems, he said.
“We can control water quality in the hatchery,” he said. “But the real question is, what happens when we take young oysters and put them in the natural environment?”
New studies show that juveniles in the natural environment experience significantly less calcification, although they’re not as vulnerable as larvae, Mook said.
Mook suggested that further research is needed in the field, the study of whether OA is changing the carbonate chemistry in shellfish grow-out systems, and whether shellfish farmers should be doing something about it.
“We need to know what the impact of acidification is on juvenile calcification rates…and look at adaptation technologies,” he said.
